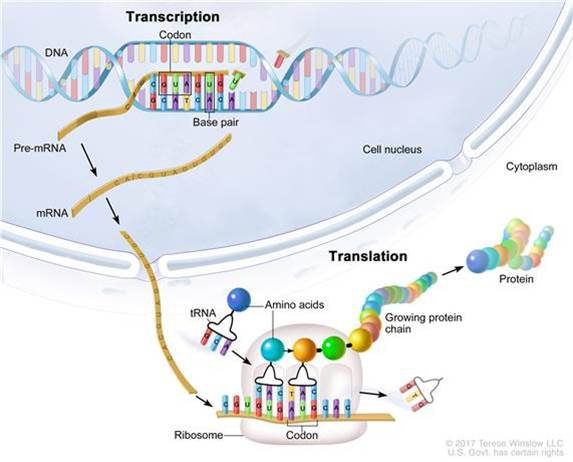
It is estimated that the prevalence of autism worldwide is 1% of the general population and increasing steadily. Males are three time more likely to affected by autism than females. Twin and family studies show that there is a genetic contribution to autism…let’s understand that more…
Is Autism Hereditary?
A study in 2017, estimated the heritability of autism to be 83%. This is slightly lower than the last study in 1995 that estimated the hereditability to be 90%. Depending on the study design, the overall risk of recurrence in siblings is estimated to be from 6.9-18%.
Single Nucleotide Polymorphisms (SNPs) account for much of the heritability, studies stay about 50%. SNPs are copy errors that happen when a cell divides. Sometimes these changes in the DNA can cause changes in appearance, disease susceptibility, etc. or absolutely nothing at all. You inherit your SNPs but also make your own.
Genome Wide association studies of large study sample size, found 93 significant genome-wise markers. 53 of those found were replicated in additional independent studies. There is a lot of genetics that is not reproducible.
In 2014, scientists concluded that rare genetic variation confers higher individual risk for autism. These are things such as small insertions or deletions, single nucleotide variations, or copy number variants. What does this mean?
The different types of genetic changes along with inherited or de novo changes define the potential genetic risk for autism. It is estimated that about 1000 genes are involved in autism. Which means that no ONE gene is likely to explain more than 1 % of cases!
What good is genetics? When presented with such diverse genetic involvement in autism, common biological mechanisms is a way to try to relate all this seemingly unrelated data. Let’s now understand…
What Really Changes With Genetic Mutations?
Missence mutations contribute to at least 10% of ASD diagnosis. Let’s quickly understand the types of mutations. Mutations are classified by the effect on DNA OR the encoded protein. It’s probably best to watch the video above because I explain silent mutations, missence mutations, nonsense mutations, and frameshift mutations using pictures. Mutations outside the coding region can also occur such as termination signals, ribosome binding sites, promoter or enhancer sites, and splice donor and acceptor sites.
Mutations are classified based on what happens to the resulting protein. When there is loss of function that means that there is either complete loss of function of the protein or reduction in the function of the protein to work. When there is gain of function that means that there is either an increase the protein’s ability to function or a new function occurs.
In 2014, it was calculated that missense mutations contribute to at least 10% of autism diagnosis. Mutations can occur in tolerant or intolerant genes, this is also important to understand. Tolerant genes carry more mutations than expected if just by chance. Intolerant genes carry fewer mutation than expected if just by chance. This is all information that scientists take into account when studying autism and genetics.
You might be thinking “What good is genetics?” When presented with such diverse genetic involvement in autism, common biological mechanisms is a way to try to relate all this seemingly unrelated data. It is estimated that about 1000 genes are involved in autism. Which means that no ONE gene is likely to explain more than 1 % of cases! So let’s examine common biological mechanisms. Next video, please.
What Common Biological Pathways Are Disrupted Due to Genetic Changes With Autism?
It is estimated that about 1000 genes are involved in autism. Which means that no ONE gene is likely to explain more than 1 % of cases! Scientists have then tried to understand if there are common biological pathways that are disrupted due to genetic changes with autism.
One type of mutation is called a nonsense mutation. Nonsense mutations cause the resulting protein to be truncated aka shortened or incomplete. Scientists studied this large genetic change since it would be easier to understand the effect and determine if there are common biological processes involved. One study revealed that the β-catenin/chromatin remodeling protein network was involved.
Beta-catenin (β-catenin) is a multifunctional protein that contributes to cell development, cell regeneration, and cell-cell adhesion. These are all biological processes that are known to not be optimal in those with autism. In “unstimulated” cells, β-catenin resides in the cytoplasm and is constantly targeted for phosphorylation and degradation.
Upon stimulation of specific signaling pathways (induced by Wnt ligands binding to Frizzled receptors), β-catenin protein is stabilized and shuttles to the nucleus, where it associates with coactivators such as T-cell factor (TCF)/lymphoid enhancer factor (LEF) family members to activate gene transcription. In the above video, I walk you through a figure of how β-catenin is used in the cell so if you are a visual learner watching the video might be best for you.
12 other De novo mutations (DNM) have been studied by various scientists and found that common biological processes involved are:
- Neuron cell-cell adhesion
- Vocalization behavior
- Glutamate receptor signaling pathway
- Cognition
- Neuron projection
In addition, other genetic studies have found that these biological processes are involved as well:
- Synaptic gene regulation (FMRP)
- Actin skeleton dynamics (TSC1/TSC2, NF1)
- Cell growth (PTEN)
- Calcium signaling (CACNA1C)
Again, you might be thinking “What good is genetic information?” Genetics is complex but thankfully there are common biological processes linking genetic information to autism. Targeting and optimizing those processes is how experts can help your child heal.
What Effect Does Paternal Age Have on Genetic Mutations in Autism?
Many parents wonder about their genetics in regards to their child’s autism. A relationship between advanced paternal age and increased autism risk has been found in several studies. De novo mutations (DNM) as well as epigenetics explain the relationship between paternal age and genetic mutations in their children. How do scientists know? DNM present in the sperm (or egg) are transmitted to the embryo therefore these mutations are present in all cells within the child. Scientists use technology to determine origin.
Most of the DNMs found associated with autism originate with the father in an age dependent manner. Scientists calculate that each additional year of paternal age result in 2 additional DNM in the child however only contributing to a 10-20% increase in autism risk.
The number of DNMs transmitted from the mother stay relatively consistent throughout the years.
Post-Zygotic Mutations (PZMs) are a type of DNMs that occur after fertilization of the egg resulting in genetically what is referred to as a mosaic individual. PZMs are implicated in several brain disorders such as epilepsy and cortical malformations as well as Rhett’s Syndrome. Detection of PZM are tricky because they are tissue-specific.
Genetics is complex but life is not necessarily dictated by genetics. The best first step in healing the body is starting the right special diet for your child. Let me explain this important aspect of genetics and to do that let’s look at some specific examples…
What Does SHANK and SCN2A Gene Mutations Do in Autism?
In this section we will look at SHANK and SCN2A and how to reverse their effects if mutated. If you’re curious about other genes, just ask in the comments below.
Mutations or disruptions in the SHANK gene family account for ~1% of those with autism. Shank proteins involved in different synaptic functions such as:
- spine morphogenesis
- synapse formation
- glutamate receptor trafficking
- activity-dependent neuronal signaling
SHANK3 mutations and duplications are associated with autism specifically causing defects in synapses maturation and function. An increased level of SHANK3 gene methylation also showed that epigenetic dysregulation of SHANK3 may be associated with autism. Here’s a quote from that scientific research paper:
“The ability to alter the epigenetic modification and expression of SHANK3 by environmental factors suggests that SHANK3 may be a valuable biomarker for dissecting the role of gene and environment interaction in the etiology of ASD.”
Let’s look at another gene. The sodium channel, voltage-gated, type II, alpha (SCN2A) gene is located on the positive strand of chromosome 2 in humans, between the sodium channel gene SCN3A and the nuclear protein gene CSRNP3. SCN2A codes for a neuronal sodium channel and is widely expressed throughout the human central nervous system but not in peripheral tissues. In cortical structures, NaV1.2 is co-expressed with NaV1.6 predominately in excitatory, glutamatergic neurons.
Loss of NaV1.2 function contributes to autism and intellectual disability, whereas gain of function contributes to early onset epilepsy. Such strong and bidirectional genotype–phenotype correlation is rare in brain disorders. Sodium channel function can be enhanced or suppressed using drugs and other environmental factors and this is how you can compensate for genetic mutations. Pharmaceutical companies are developing drugs but the same effect can be done with environmental factors.
It is estimated that about 1000 genes are involved in autism.
Which means that no ONE gene is likely to explain more than 1% of cases!
Genetics is complex but life is not necessarily dictated by genetics. The best first step in healing the body is starting the right special diet for your child. Control the environmental factors.